
1. Choose the appropriate option and rewrite the following statements:
a.The intermolecular force is ……………. in the particles of solid.
(a) minimum
(b) moderate
(c) maximum
(d) indefinite
Answer:
(c) maximum
b.Solids retain their volume even when external pressure is applied. This property is called …………… .
(a) plasticity
(b) incompressibility
(c) fluidity
(d) elasticity
Answer:
(d) elasticity
c. Matter is classified into the types mixture, compound and element, by applying the criterion …………… .
(a) states of matter
(b) phases of matters
(c) chemical composition of matter
(d) all of these
Answer:
(c) chemical composition of matter
d. Matter that contain two or more constituent substances is called ……… .
(a) mixture
(b) compound
(c) element
(d) metalloid
Answer:
(a) mixture
e. Milk is an example of type of matter called …………. .
(a) solution
(b) homogeneous mixture
(c) heterogeneous mixture
(d) suspension
Answer:
(c) heterogeneous mixture
f. Water, mercury and bromine are similar to each other, because the three are ……….. .
(a) liquids
(b) compounds
(c) nonmetals
(d) elements
Answer:
(a) liquids
g. Valency of carbon is 4 and that of oxygen is 2. From this, we understand that there are …………. chemical bond/bonds between the carbon atom and one oxygen atom in the compound- carbon dioxide.
(a) 1
(b) 2
(c) 3
(d) 4.
Answer:
(b) 2
2. Identify the odd term out and explain.
a. Gold, silver, copper, brass.
Answer:
Brass. (Others are elements.)
b. Hydrogen, hydrogen peroxide, carbon dioxide, water vapour.
Answer:
Hydrogen. (Others are compounds.)
c. Milk, lemon juice, carbon, steel.
Answer:
Carbon. (Others are mixtures.)
d. Water, mercury, bromine, petrol.
Answer:
Petrol. (Others are inorganic compounds.)
e. Sugar, salt, baking soda, blue vitriol.
Answer:
Sugar. (Others are inorganic compounds.)
f. Hydrogen, sodium, potassium, carbon.
Answer:
Carbon. (Others are monovalent elements)

3. Answer the following question.
a. Plants synthesize glucose in sunlight with the help of chlorophyll from carbon dioxide and water and give away oxygen. Identify the four compounds in this process and name their types.
Answer:
Photosynthesis:
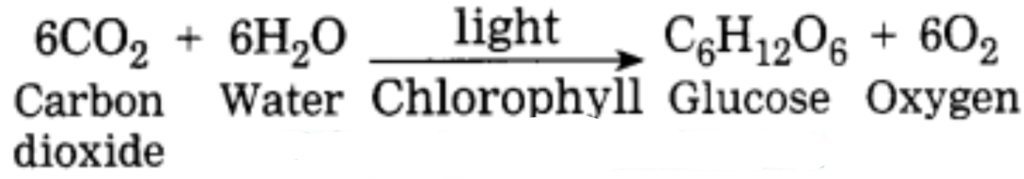
Carbon dioxide, water, glucose, and Chlorophyll are compounds.
Types: Organic compounds: Glucose
Inorganic compounds: Carbon dioxide and water
Complex compounds: Chlorophyll.
b. In one sample of brass, the following ingredients were found: copper (70%) and zinc (30%). Identify the solvent, solute and solution from these.
Answer:
Brass is an alloy, it contains 70% copper and 30% zinc. The largest proportion is solvent, i.e. copper. The smaller proportion is solute, i.e. zinc. The solution is Brass.
c. Seawater tastes salty due to the dissolved salt. The salinity (the proportion of salts in water) of some water bodies Lonar lake – 7.9%, Pacific Ocean 3.5%, Mediterranean sea – 3.8%, 5 Dead sea – 33.7%. Explain two characteristics of mixtures from the above information.
Answer:
- The constituents of a mixture (the proportion of salts in water) do not combine chemically.
- Their constituents are present in any proportion by weight.
- The constituent of a mixture can be separated by a physical process.
4. Give two examples each.
a. Liquid element.
Answer:
Mercury (Hg), Bromine (Br2)
b. Gaseous element.
Answer:
Hydrogen (H2), Oxygen (O2).
c. Solid element.
Answer:
Iron (Fe), Copper (Cu), Silver (Ag).
d. Homogeneous mixture.
Answer:
Sea water, blue vitriol dissolved in water.
e. Colloid.
Answer:
Milk, blood.
f. Organic compound.
Answer:
Glucose, urea.
g. Complex compound.
Answer:
Chlorophyll, Haemoglobin.
h. Inorganic compound.
Answer:
Soda, rust, limestone.
i. Metalloid.
Answer:
Silicon, arsenic.
j. Element with valency 1.
Answer:
Sodium (Na), potassium (K), chlorine (Cl).
l. Element with valency 2.
Answer:
Magnesium (Mg), Calcium (Ca).
5. Write the names and symbols of the constituent elements and identify their valencies from their molecular formulae given below:
KCl, HBr, MgBr2. K2O, NaH, CaCl2, CCl4, HI, H2S, Na2S, FeS, BaCl2.
Answer:
| Molecular formula | Constituent element/Name symbol | Valency |
| 1. KCl | Potassium (K) Chlorine (Cl) | 1 1 |
| 2. HBr | Hydrogen (H) Bromine (Br) | 1 1 |
| 3. MgBr2 | Magnesium (Mg) Bromine (Br) | 2 1 |
| 4. K2O | Potassium (K) Oxygen (O) | 1 2 |
| 5. NaH | Sodium (Na) Hydrogen (H) | 1 1 |
| 6. CaCl2 | Calcium (Ca) Chlorine (Cl) | 2 1 |
| 7. CCl4 | Carbon (C) Chlorine (Cl) | 4 1 |
| 8. HI | Hydrogen (H) Iodine (I) | 1 1 |
| 9. H2S | Hydrogen (H) Sulphur (S) | 1 2 |
| 10. Na2S | Sodium (Na) Sulphur (S) | 1 2 |
| 11. FeS | Iron (Fe) Sulphur (S) | 2 2 |
| 12. BaCl2 | Barium (Ba) Chlorine (Cl) | 2 1 |
6. Chemical composition of some matter is given in the following table. Identify the main type of matter from them.
a. Chemical composition of some matter is given in the following table. Identify the main type of matter from them.
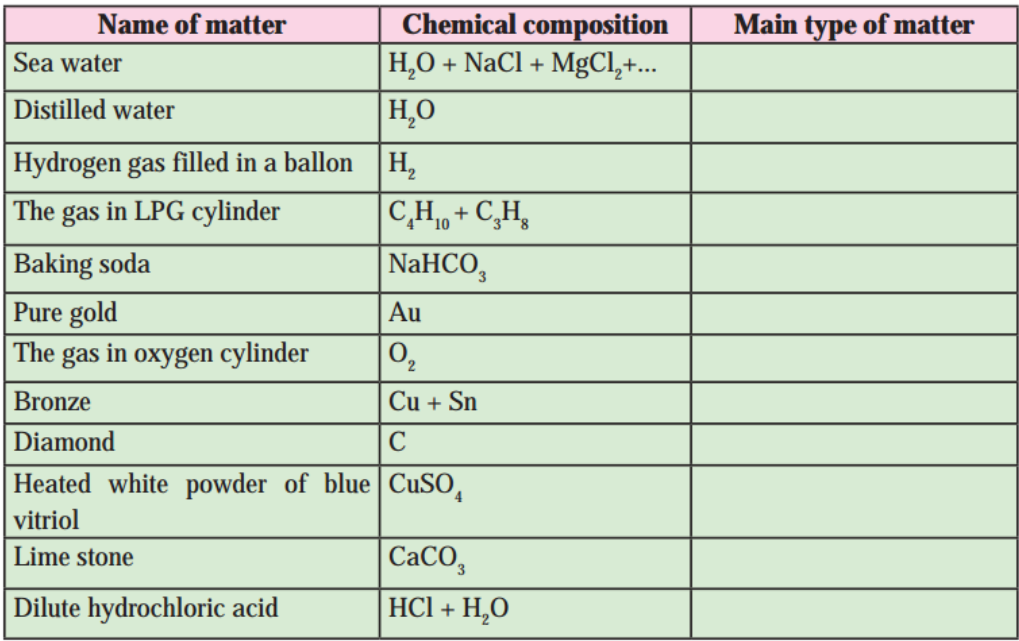
Answer:
| Name of matter | Chemical composition | Main type of matter |
| Sea water | H2O + NaCl + MgCl2 +… | Mixture |
| Distilled water | H2O | Compound |
| Hydrogen gas filled in a balloon | H2 | Element |
| The gas in LPG cylinder | C4H10 + C3H8 | Mixture |
| Baking soda | NaHCO3 | Compound |
| Pure gold | Au | Element |
| The gas in oxygen cylinder | O2 | Element |
| Bronze | Cu + Sn | Mixture |
| Diamond | C | Element |
| Heated white powder of blue vitriol | CuSO4 | Compound |
| Limestone | CaCO3 | Compound |
| Dilute hydrochloric acid | HCl + H2O | Mixture |
7. Write scientific reason.
a. Hydrogen is combustible, oxygen helps combustion, but water helps to extinguish fire.
Answer:
- Water is a compound of hydrogen and oxygen.
- In a compound, the constituents do not retain their individual properties. Hence, hydrogen is combustible and oxygen helps combustion, but water is neither combustible nor supports combustion, it helps to extinguish fire.
b. The constituent substances of a colloid cannot be separated by oridinary filtration.
Answer:
- A colloidal solution is heterogeneous.
- The diameters of colloidal particles are of the order of 10-5 m.
- The particles of a colloid can easily pass through a filter paper as the pore size of a filter paper is big. Hence, the constituents of a colloidal cannot be separated by filtration.
c. Lemon sherbat has sweet, sour and salty taste and it can be poured in a glass.
Answer:
- Lemon sherbat is a mixture. It is made up of lemon juice, sugar, salt and water.
- Formation of lemon sherbat does not involve any chemical reaction.
- The constituents of sherbat retain their individual properties. Hence, lemon sherbat is sweet, sour and salty to taste and it can be poured in a glass.
d. A solid matter has the properties of definite shape and volume.
Answer:
- The forces among the constituent particles (atom/molecules) are called intermolecular forces.
- In solids these forces are strong enough to keep the particles together in fixed positions, as a result solids have a definite shape and volume.
8. Deduce the molecular formulae of the compound obtained from the following pairs of elements by the cross multiplication method.
a. C (Valency 4) & Cl (Valency 1)
Answer:
Step 1: Write the symbols of the constituent elements.![]()
Step 2: Write the valency below the respective elements.![]()
Step 3: Cross-multiply the valencies.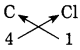
The molecular formula: CCl4
b. N (Valency 3) & H (Valency 1)
Answer:
Step 1: Write the symbols of the constituent elements.![]()
Step 2: Write the valency below the respective elements.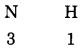
Step 3: Cross-multiply the valencies.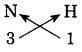
The molecular formula: NH3
c. C (Valency 4) & O (Valency 2)
Answer:
Step 1: Write the symbols of the constituent elements.![]()
Step 2: Write the valency below the respective elements.![]()
Step 3: Cross multiply the valencies.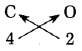
The formula: C2O4
The number of constituent atoms in the final molecular formula should be the smallest possible whole number. Divide the formula C2O4 by suitable number.
Final molecular formula obtained by dividing by ‘2’.
Molecular formula: CO2
d. Ca (Valency 2) & O (Valency 2)
Answer:
Step 1: Write the symbols of the constituent elements.![]()
Step 2: Write the valency below the respective elements.![]()
Step 3: Cross multiply the valencies.![]()
The formula: Ca2O2
Divide the formula by suitable number ‘2’.
The molecular formula: CaO.


