
1. Complete the statement by filling the gaps using appropriate term from the terms given in the brackets:
(slow, coloured, arrow, fast, smell, milky, physical, product, chemical, reactant, covalent, ionic, octet, duplet, exchange, sharing, equality sign)
a. An ……….. is drawn in between the reactants and products while writing the equation for a chemical reaction.
Answer:
An arrow is drawn in between the reactants and products while writing the equation for a chemical reaction.
b. Rusting of iron is a ……….. chemical change.
Answer:
Rusting of iron is a slow chemical change.
c. The spoiling of food is a chemical change which is recognized from the generation of certain …………. due to it.
Answer:
The spoiling of food is a chemical change which is recognized from the generation of certain smell due to it.
d. A colourless solution of calcium hydroxide in a test tube turns ………….. on blowing in it through a blow tube for some time.
Answer:
A colourless solution of calcium hydroxide in a test tube turns milky on blowing in it through a blow tube for some time.
e. The white particles of baking soda disappear when put in lemon juice. This means that it is a ……….. change.
Answer:
The white particles of baking soda disappear when put in lemon juice. This means that it is a chemical change.
f. Oxygen is a …………….. in respiration.
Answer:
Oxygen is a reactant in respiration.
g. Sodium chloride is …………… compound while hydrogen chloride is compound.
Answer:
Sodium chloride is ionic compound while hydrogen chloride is covalent compound.
h. Electron …………….. is complete in each hydrogen in a hydrogen molecule.
Answer:
Electron duplet is complete in each hydrogen in a hydrogen molecule.
I. Chlorine (Cl2) molecule is formed by ………….. of electrons between two chlorine atoms.
Answer:
Chlorine (Cl2) molecule is formed by sharing of electrons between two chlorine atoms.
2. Explain by writing a word equation.
a. Respiration is a chemical change.
Answer:
Respiration is a biological process, in this process air is inhaled, oxygen present in this inhaled air reacts with glucose present in the cells of the body forming carbon dioxide and water. Moreover, we cannot obtain glucose and oxygen from carbon dioxide and water. Hence, respiration is a chemical change.
Word equation:

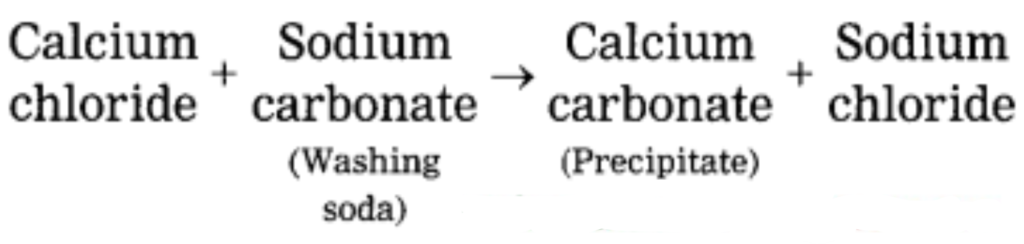
b. Hard water gets softened on mixing with a solution of washing soda.
Answer:
Hard water does not form lather with soap and is brackish to taste. This is because hard water contains the chloride and sulphate salts of calcium and magnesium in dissolved state. When a solution of washing soda is added to hard water, it forms a precipitate of calcium carbonate and magnesium carbonate, which is removed by filtration thus water is softened.
Word equation:
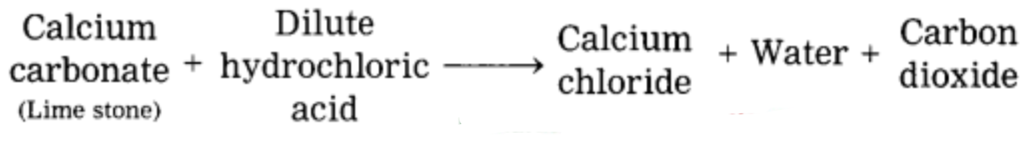
c. Limestone powder disappears on adding to dilute hydrochloric acids.
Answer:
In the reaction of dil. HCl and limestone powder (CaCO3), limestone disappears slowly and carbon dioxide (CO2) liberates slowly.
Word equation:
d. Bubbles are seen on adding lemon juice to baking soda.
Answer:
When baking soda is added to lemon juice a chemical change takes place in citric acid present in the lemon juice and carbon dioxide gas is formed. Word equation:

This is a neutralization reaction.
3. Match the pairs.
| Column I | Column II |
| 1. Photosynthesis | a. Tendency to lose electrons |
| 2. Water | b. Reactant in combustion process |
| 3. Sodium chloride | c. Chemical change |
| 4. Dissolution of salt in water | d. Covalent bond |
| 5. Carbon | e. Ionic bond |
| 6. Fluorine | f. Physical change |
| 7. Magnesium | g. Tendency to form anion |
Answer:
| Column I | Column II |
| 1. Photosynthesis | c. Chemical change |
| 2. Water | d. Covalent bond |
| 3. Sodium chloride | e. Ionic bond |
| 4. Dissolution of salt in water | f. Physical change |
| 5. Carbon | b. Reactant in combustion process |
| 6. Fluorine | g. Tendency to form anion |
| 7. Magnesium | a. Tendency to lose electrons |

4. Show with the help of diagram of electronic configuration how the following compounds are formed from the constituent atoms.
a. Sodium Chloride:
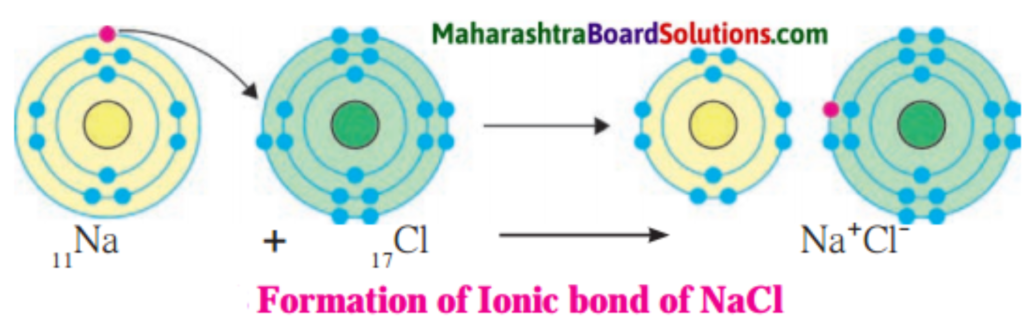
Answer:
1. Sodium has atomic number 11 and electronic configuration 2, 8, 1.
2. Sodium atom has 1 electron in its outermost shell.
3. It loses one electron from its outermost shell, i.e., M shell. Then its L shell becomes the outermost shell with a stable octet. The nucleus of sodium atom has 11 protons but the number of electrons in the atom has become 10. So, there is a net unit positive charge giving a sodium cation (Na+).
4. On the other hand, chlorine has electronic configuration 2, 8, 7. Chlorine atom has 7 electrons in its outermost shell and requires one electron to complete its octet.
5. Thus, the electron lost by sodium is taken up by chlorine.
6. When chlorine atom gains one electron, octet of chlorine is completed and its K, L, M shells have together 18 electrons and the nucleus has 17 protons. This leads to the formation of an ion (CP).
7. Thus, a chlorine atom accepts one electron from a sodium atom and consequently a chloride ion with one unit negative charge and a sodium ion with one unit positive charge are formed.
8. Sodium and chloride ions, being oppositely charged, attract each other due to the electrostatic force of attraction. An ionic bond is formed and this results in the formation of sodium chloride (NaCl) molecule.
b. Potassium fluoride:
Answer:
1. Potassium has atomic number 19 and electronic configuration 2, 8, 8, 1.
2. Potassium atom has 1 electron in its outermost shell.
3. It loses one electron from its outermost shell, i.e. N shell. Then its M shell becomes the outermost shell with a stable octet. The nucleus of potassium atom has 19 protons but the number of electrons in the atom has become 18. So there is a net unit positive charge giving a potassium cation (K+).
4. On the other hand, fluorine has electronic configuration 2, 7. Fluorine has 7 electrons in the outermost shell and requires one electron to complete its octet.
5. Thus, the electron lost by potassium is taken up by chlorine.

6. When fluorine atom gains one electron, octet of fluorine is completed, its K and L shells have together 10 electrons and the nucleus has 9 protons. This leads to the formation of an ion (F–).
7. Thus, a fluorine atom accepts one electron from a potassium atom and consequently a fluoride ion with one unit negative charge and a potassium ion with one unit positive charge are formed.
8. Potassium and fluoride ions, being oppositely charged, attract each other due to electrostatic force of attraction. An ionic bond is formed and this results in the formation of potassium fluoride (KF) molecule.
c. Water:
Answer:
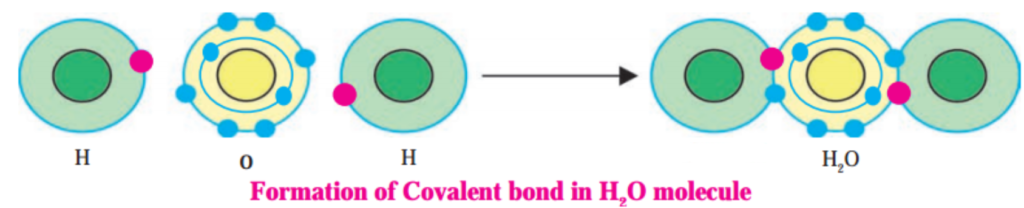
1. Hydrogen has atomic number 1 and electronic configuration 1.
2. Hydrogen has 1 electron in its K shell.
3. Oxygen has atomic 8 and electronic configuration 2, 6. There are 6 electrons in the valence shell of oxygen atom. It means that the electron octet in oxygen is short of two electrons and the valency of oxygen is two.
4. In the H2O molecule, the oxygen atom complete its octet by sharing two electrons one each with two hydrogen atoms, thus, forming two covalent bonds. While this happens, the duplets of two hydrogen atoms are completed.
d. Hydrogen chloride:
Answer:
1. Hydrogen has atomic number 1 and electronic configuration 1, that means it has 1 electron in its K shell and its duplet is short of one electron therefore, the valency of hydrogen is one.
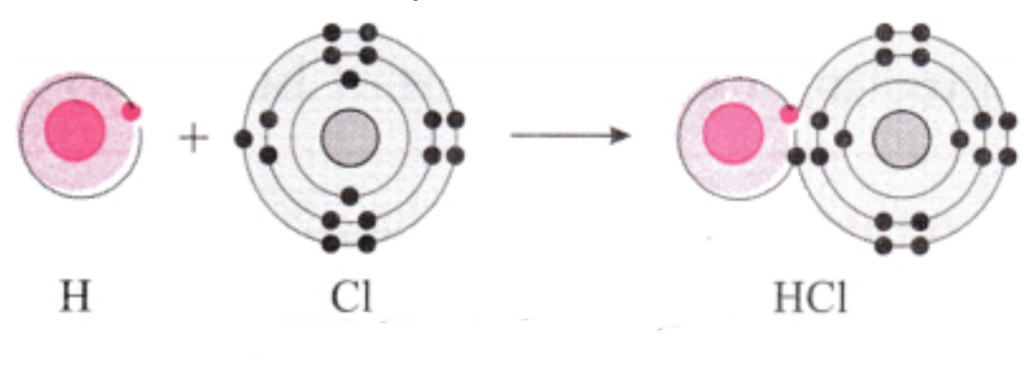
2. On the other hand, chlorine has electronic configuration 2, 8, 7. Chlorine atom has 7 electrons in its outermost shell and requires one electron to complete its octet.
3. The two atoms, hydrogen and chlorine share one electron with each other. As a result, the electron duplet of hydrogen and octet of chlorine is complete and a covalent band is formed between them.


