1. Identify the odd one out and justify.
(a) Chloride, nitrate, hydride, ammonium
Answer.
Ammonium is the odd one out as it is a basic radical and rest all are acidic radicals. Generally, basic radicals are formed by the removal of electrons from the atom of metals such as Na+, Cu2+. But there are some exceptions, such as NH4+.
(b) Hydrogen chloride, sodium hydroxide, calcium oxide, ammonia
Answer.
Hydrogen chloride is the odd one out. It is acidic and rest all are basic.
(c) Acetic acid, carbonic acid, hydrochloric acid, nitric acid
Answer.
Carbonic acid is the odd one out. It is a dibasic acid and rest are all monobasic acids.
(d) Ammonium chloride, sodium chloride, potassium nitrate, sodium sulphate
Answer.
Ammonium chloride is the odd one out, as it is made up of a strong acid and weak base and rest all are formed from strong acid and strong base.
(e) Sodium nitrate, sodium carbonate, sodium sulphate, sodium chloride
Answer.
Sodium carbonate is the odd one out, as it is made up of a weak acid and strong base, and rest all are formed from strong acid and strong base.
(f) Calcium oxide, magnesium oxide, zinc oxide, sodium oxide.
Answer.
Zinc oxide is the odd one out, as it is an amphoteric oxide, and rest all are basic oxides.
(g) Crystalline blue vitriol, crystalline common salt, crystalline ferrous sulphate, crystalline sodium carbonate.
Answer.
Crystalline common salt is the odd one out, as it does not contain water of crystallization. It is an ionic compound and ionic compounds are crystalline in nature and rest all have their crystalline structure because of their water of crystallization.
(h) Sodium chloride, potassium hydroxide, acetic acid, sodium acetate
Answer.
Acetic acid is the odd one out. It is an acid, the rest are all salts.

2.Write down the changes that will be seen in each instance and explain the reason behind it.
(a) 50ml water is added to 50ml solution of copper sulphate
Answer.
- Copper sulphate solution is blue. It is a concentrated solution.
- When 50 ml of water is added to this concentrated solution, it becomes a diluted solution.
- The intensity of the blue colour is now different in this homogenous mixture.
(b) Two drops of the indicator phenolphthalein were added to 10ml solution of sodium hydroxide.
Answer.
- Sodiumhy droxide is a base and phenolphthalein is a synthetic indicator.
- Sodium hydroxide solution will turn pink if phenolphthalein is added to it.
- It is a test for identifying bases.
(c) Two or three filings of copper were added to 10ml dilute nitric acid and stirred.
Answer.
When two or three filings of copper were added to 10 ml dilute nitric acid, it forms copper nitrate and hydrogen gas.
(d) A litmus paper was dropped into 2ml dilute HCl. Then 2ml concentrated NaOH was added to it and stirred.
Answer.
A piece of litmus paper was added into 2 ml dilute HCl, blue litmus turns red. When in the same solution 2 ml concentrate NaOH was added and stirred, then the red litmus turned blue.
(e) Magnesium oxide was added to dilute HCl and magnesium oxide was added to dilute NaOH.
Answer.
(1) When magnesium oxide was added to dilute HCl, it forms magnesium chloride and water. Magnesium oxide being basic in nature it neutralizes acid.
(2) There is no reaction between magnesium oxide and dilute NaOH, as both are basic in nature.
(f) Zinc oxide was added to dilute HCl and zinc oxide was added to dilute NaOH.
Answer.
When zinc oxide was added to dilute HCl, it forms zinc chloride and water. In this reaction, zinc oxide is a basic oxide. When zinc oxide was added to dilute NaOH, it forms sodium zincate and water. In this reaction, zinc oxide is an acidic oxide. Therefore, zinc oxide is an amphoteric oxide because it shows both acidic and basic properties.
(g) Dilute HCl was added to limestone
Answer.
When dilute HCl was added to lime stone, it forms calcium chloride, water and carbon dioxide gas.
(h) Pieces of blue vitriol were heated in a test tube. On cooling, water was added to it.
Answer.
When pieces of blue vitriol were heated in a test tube, the crystalline structure of blue vitriol broke down to form colourless powder and water
evaporates. On cooling when water was added to colourless powder, blue vitriol regains its blue colour. All the above changes are physical changes.
(i) Dilute H2SO4 was taken in an electrolytic cell and electric current was passed through it.
Answer.
When electric current was passed through dilute H2SO4 in an electrolytic cell, H2 gas was formed at the cathode and O2 gas was formed at the anode.
3. Classify the following oxides into three types and name the types.
CaO, MgO, CO2, SO3, Na2O, ZnO, Al2O3, Fe2O3
Answer.
(1) Basic oxides : CaO, MgO, Na2O, Fe2O3
(2) Acidic oxides : CO2, SO3
(3) Amphoteric oxides : ZnO, Al2O3
4. Explain by drawing a figure of the electronic configuration.
a. Formation of sodium chloride from sodium and chlorine.
Answer.
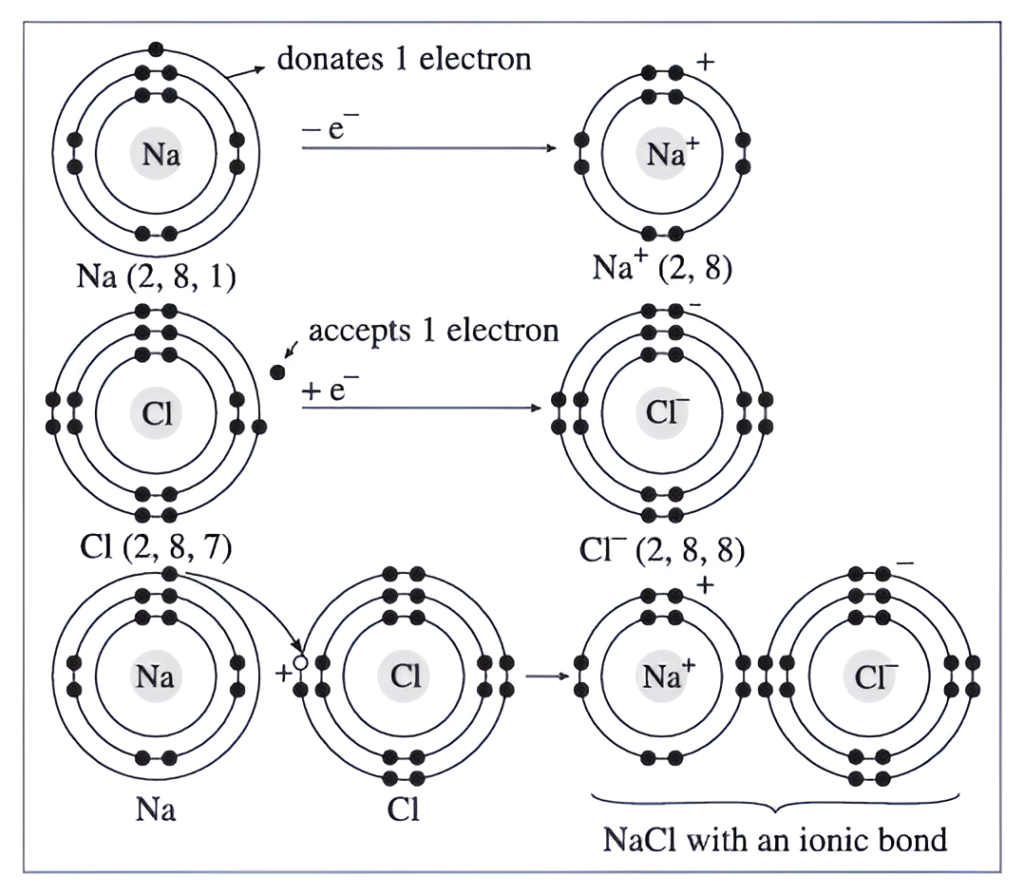
(1) An atom of sodium has one electron in its outermost orbit.
(2) An atom of chlorine has seven electrons in its outermost orbit.
(3) When these two atoms come close together, the sodium atom donates its electron and the chlorine atom accepts it, thus, both acquire octet state.
(4) Due to this, the sodium and chlorine atoms become positive and negative ions respectively. This results in the formation of an ionic bond between the two ions, due to electrostatic force of attraction, giving rise to ionic compound sodium chloride.
b. Formation of magnesium chloride from magnesium and chlorine.
Answer.
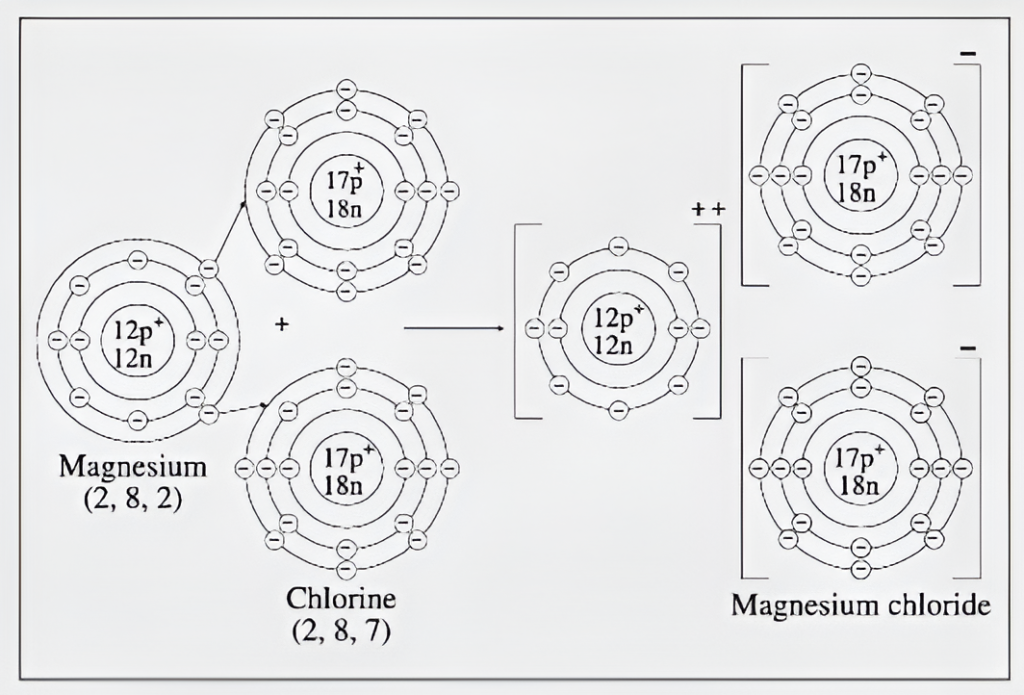
(1) The magnesium atom has 2 electrons in its outermost orbit. It gives electrons from its outermost orbit and gets converted into a positive ion with 2 unit positive charge.
(2) The chlorine atom has 7 electrons in its outermost orbit. So, each chlorine atom needs only one electron to establish the octet state of its outermost orbit and thereby gets converted into a negatively charged chloride ion with a unit negative charge.
(3) So two chlorine atoms accept one electron each from a magnesium atom and consequently two chloride ions and a magnesium ion are formed.
(4) Due to the electrostatic force of attraction an ionic bond is formed and this results in the formation of magnesium chloride molecule.
5. Show the dissociation of the following compounds on dissolving in water, with the help of chemical equation and write whether the proportion of dissociation is small or large.
Hydrochloric acid, Sodium chloride, Potassium hydroxide, Ammonia, Acetic acid, Magnesium chloride, Copper sulphate.
Answer.
Dissociation of Compounds in Water (Class 9 Science)
| Compound | Chemical Equation (Dissociation) | Proportion of Dissociation | Nature |
|---|---|---|---|
| Hydrochloric acid | HCl → H+ + Cl– | Large | Strong Acid |
| Sodium chloride | NaCl → Na+ + Cl– | Large | Salt |
| Potassium hydroxide | KOH → K+ + OH– | Large | Strong Base |
| Ammonia | NH3 + H2O ⇌ NH4+ + OH– | Small | Weak Base |
| Acetic acid | CH3COOH ⇌ CH3COO– + H+ | Small | Weak Acid |
| Magnesium chloride | MgCl2 → Mg2+ + 2Cl– | Large | Salt |
| Copper sulphate | CuSO4 → Cu2+ + SO42- | Large | Salt |
6. Write down the concentration of each of the following solutions in g/L and mol/L.
a. 7.3g HCl in 100ml solution
b. 2g NaOH in 50ml solution
c. 3g CH3COOH in 100ml solution
d. 4.9g H2SO4 in 200ml solution
Answer.
| Solute | Quantity of solute | Volume of Solution | Concentration of the solution | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E = D/C | F | G = D/F | H = E/F |
| Name | Molecular formula | Molecular mass (u) | Gram (g) | Mole (mol) | Litre (L) | Gram/Litre (g/L) | Molarity (M) (mol/L) |
| Hydrochloric acid | HCl | 36.5 u | 7.3 g | 0.2 mol | 100 ml = 0.1L | 73 g/L | 2 mol/L |
| Sodium Hydroxide | NaOH | 40 u | 2.0 g | 0.05 mol | 50 ml = 0.05L | 40 g/L | 1 mol/L |
| Acetic Acid | CH3COOH | 60 u | 3.0 g | 0.05 mol | 100 ml = 0.1L | 30 g/L | 0.5 mol/L |
| Sulphuric acid | H2SO4 | 98 u | 4.9 g | 0.05 mol | 200 ml = 0.2L | 24.5 g/L | 0.25 mol/L |
7. Answer the following questions
a. Classify the acids according to their basicity and give one example of each type.
Answer.
- Basicity of acids : The number of H+ ions obtainable by the dissociation of one molecule of an acid is called its basicity. The acids are classified as monobasic, dibasic and tribasic acids based on the number of H+ ions present.
- Examples of monobasic acid : HCl, HNO3, CH3COOH
- Examples of dibasic acid: H2SO4, H2CO3
- Examples of tribasic acid: H3BO3, H3PO4
b. What is meant by neutralization? Give two examples from everyday life of the neutralization reaction.
Answer.
- In neutralization reaction, an acid reacts with a base to form salt and water.
- In a neutralisation reaction the acid dissociates to form H+ ions and base dissociates to form OH ions.
- They combine to form H2O molecules which mixes with the solvent.
Examples in daily life:
- When people suffer from acidity, they take some antacids to neutralise the acid in their stomach.
- If an ant stings us the pain is due to formic acid. It is neutralised by rubbing moist baking soda which is basic in nature.
c. Explain what is meant by electrolysis of water. Write the electrode reactions and explain them.
Answer.
Electrolysis of Water
Electrolysis of water is the process of using electrical energy to decompose water (H2O) into its constituent elements: Hydrogen gas (H2) and Oxygen gas (O2).
Since pure water is a non-conductor of electricity, a few drops of dilute Sulphuric acid (H2SO4) are added to make it a conductor (acidulated water).
Electrode Reactions
1. At the Cathode (Negative Electrode)
Reduction occurs at the cathode. Hydrogen ions or water molecules gain electrons to form hydrogen gas.
Explanation: Electrons are supplied by the battery to the cathode. Water molecules accept these electrons, releasing Hydrogen gas. This gas is collected at the cathode.
2. At the Anode (Positive Electrode)
Oxidation occurs at the anode. Water molecules lose electrons to form oxygen gas.
Explanation: Water molecules give up electrons to the positive electrode. This produces Oxygen gas and releases H+ ions into the solution.
Key Observations
| Feature | Cathode (-) | Anode (+) |
|---|---|---|
| Gas Collected | Hydrogen (H2) | Oxygen (O2) |
| Volume Ratio | 2 Parts | 1 Part |
| Reaction Type | Reduction | Oxidation |
Why is the volume of Hydrogen double? According to the chemical formula H2O, water contains two atoms of Hydrogen for every one atom of Oxygen. Therefore, during electrolysis, the volume of hydrogen gas evolved is exactly double the volume of oxygen gas.
8.Write the chemical equations for the following activities.
(a) NaOH solution was added to HCl solution.
(b) Zinc dust was added to dilute H2SO4.
(c) Dilute nitric acid was added to calcium oxide.
(e) Carbon dioxide gas was passed through KOH solution.
(f) Dilute HCl was poured on baking soda.
Answer.
Chemical Equations (Class 9 Science)
9. State the differences.
a. Acids and bases
Answer.
| Acids | Bases |
| (i) A substance which liberates H+ ions when dissolved in water is an acid (ii) Blue litmus turns red in an acid. (iii) The pH of an acid is less than 7. (iv) Acids are sour to taste (v) e.g. HCl, H2SO4 | A substance which liberates OH– ions when dissolved in water is called a base. Red litmus turns blue in a base The pH of a base is greater than 7. Bases are bitter to taste, e.g. NaOH, KOH. |
b. Cation and anion
Answer.
| Cations | Anions |
| (i) Cations are ions with a net positive charge. | Anions are ions with a net negative charge. |
| (ii) Cations are generally formed by metals. When metals donate electrons, they have excess of protons, hence they form cations. | Anions are generally formed by non-metals. When non-metals accept electrons, they have excess of electrons, hence they form anions. |
| (iii) Cations are attracted towards the cathode which are negatively charged electrodes. | Anions are attracted towards the anode which are positively charged electrodes. |
| (iv) e.g.: Na+, Ca2+, Mg2+, K+ etc. | e.g.: O2 , S2-, Cl–, Br– etc. |
c. Negative electrode and positive electrode.
Answer.
| Negative Electrode | Positive Electrode |
| (i) Negatively charged electrodes are called as a cathode. | Positively charged electrodes are called as Anode. |
| (ii) Positively charged cations move towards the cathode or negative electrode. | Negatively charged anions move towards the anode or positive electrode. |
| (iii) Cathode accepts electrons from cations | Anode gives electrons to anions |
10. Classify aqueous solutions of the following substances according to their pH into three groups : 7, more than 7, less than 7.
Common salt, sodium acetate, hydrochloric acid, carbon dioxide, potassium bromide, calcium hydoxide, ammonium chloride, vinegar, sodium carbonate, ammonia, sulphur dioxide.
Answer.
| Solute | Quantity of solute | Volume of Solution | Concentration of the solution | ||||
| A | B | C | D | E = D/C | F | G = D/F | H = E/F |
| Name | Molecular formula | Molecular mass (u) | Gram (g) | Mole (mol) | Litre (L) | Gram/Litre (g/L) | Molarity (M) (mol/L) |
| Hydrochloric acid | HCl | 36.5 u | 7.3 g | 0.2 mol | 100 ml = 0.1L | 73 g/L | 2 mol/L |
| Sodium Hydroxide | NaOH | 40 u | 2.0 g | 0.05 mol | 50 ml = 0.05L | 40 g/L | 1 mol/L |
| Acetic Acid | CH3COOH | 60 u | 3.0 g | 0.05 mol | 100 ml = 0.1L | 30 g/L | 0.5 mol/L |
| Sulphuric acid | H2SO4 | 98 u | 4.9 g | 0.05 mol | 200 ml = 0.2L | 24.5 g/L | 0.25 mol/L |


